Reaksi Ionisasi Larutan Elektrolit kimia
Reaksi Ionisasi Larutan Elektrolit kimia
1. Elektrolit Kuat
a. Asam kuat
HxZ (aq)-> x H+(aq) + Zx–(aq)
Contoh:
• HCl(aq)-> H+(aq) + Cl–(aq)
• H2SO4(aq)-> 2 H+(aq) + SO42–(aq)
• HNO3(aq)-> H+(aq) + NO3–(aq)
b. Basa kuat
M(OH)x(aq)-> Mx+(aq) + x OH–(aq)
Contoh:
• NaOH(aq)-> Na+(aq) + OH–(aq)
• Ba(OH)2(aq)-> Ba2+(aq) + 2 OH–(aq)
• Ca(OH)2(aq)-> Ca2+(aq) + 2 OH–(aq)
c. Garam
MxZy(aq)-> x My+(aq) + y Zx–(aq)
Contoh:
• NaCl(aq)-> Na+(aq) + Cl–(aq)
• Na2SO4(aq)-> 2 Na+(aq) + SO42–(aq)
• Al2(SO4)3(aq)-> 2 Al3+(aq) + 3SO42–(aq)
2. Elektrolit Lemah
a. Asam lemah
HxZ(aq)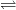 x H+(aq) + Zx–(aq)
x H+(aq) + Zx–(aq)
Contoh:
CH3COOH(aq)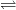 H+(aq) + CH3COO–(aq)
H+(aq) + CH3COO–(aq)
H2SO3(aq)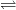 2 H+(aq) + SO32–(aq)
2 H+(aq) + SO32–(aq)
H3PO4(aq)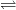 3 H+(aq) + PO4–(aq)
3 H+(aq) + PO4–(aq)
b. Basa lemah
M(OH)x(aq)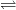 Mx+(aq) + x OH–(aq)
Mx+(aq) + x OH–(aq)
Contoh:
NH4OH(aq)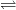 NH4+(aq) + OH–(aq)
NH4+(aq) + OH–(aq)
Al(OH)3(aq)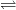 Al3+(aq) + 3 OH–(aq)
Al3+(aq) + 3 OH–(aq)
Fe(OH)2(aq)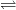 Fe2+(aq) + 2 OH–(aq)
Fe2+(aq) + 2 OH–(aq)
Literatur/Refrensi Artikel: Cham-is-try.org
1. Elektrolit Kuat
a. Asam kuat
HxZ (aq)-> x H+(aq) + Zx–(aq)
Contoh:
• HCl(aq)-> H+(aq) + Cl–(aq)
• H2SO4(aq)-> 2 H+(aq) + SO42–(aq)
• HNO3(aq)-> H+(aq) + NO3–(aq)
b. Basa kuat
M(OH)x(aq)-> Mx+(aq) + x OH–(aq)
Contoh:
• NaOH(aq)-> Na+(aq) + OH–(aq)
• Ba(OH)2(aq)-> Ba2+(aq) + 2 OH–(aq)
• Ca(OH)2(aq)-> Ca2+(aq) + 2 OH–(aq)
c. Garam
MxZy(aq)-> x My+(aq) + y Zx–(aq)
Contoh:
• NaCl(aq)-> Na+(aq) + Cl–(aq)
• Na2SO4(aq)-> 2 Na+(aq) + SO42–(aq)
• Al2(SO4)3(aq)-> 2 Al3+(aq) + 3SO42–(aq)
2. Elektrolit Lemah
a. Asam lemah
HxZ(aq)
Contoh:
CH3COOH(aq)
H2SO3(aq)
H3PO4(aq)
b. Basa lemah
M(OH)x(aq)
Contoh:
NH4OH(aq)
Al(OH)3(aq)
Fe(OH)2(aq)
Literatur/Refrensi Artikel: Cham-is-try.org
0 comments:
Posting Komentar